How to Produce Billions of Years in just 30 minutes?
The Paradox of Morphological Stasis, the Abolition of Conventional Geochronology and the New Neocatastrophic Impact Theory
ARTIGO ORIGINAL EM PORTUGUÊS : https://sodregoncalves.no.comunidades.net/mudancas-morfologicas-em-tempo-geologico
Last revision Diluvio de Pedras
Sodré, GBN; Alves, EE; Luther, BS; Barna, D; Machado, RC
Sodré Gonçalves de Brito Neto¹, Everton Fernando Alves²
¹Graduating in Geology, Federal University of Goiás, UFG, Brazil.
²Department of Biotechnology, Genetics and Cell Biology, State University of Maringá, UEM, Paraná, Brazil.
³Department of General Pathology Federal University of Minas Gerais, UFMG, Belo Horizonte, Brazil.
Hector Luther Honorato de Brito Siman, Dan de Barna, author of the book
Roberto Carlos Machado independent researcher
ABSTRACT - The anomaly surprisingly found that there are no significant morphological changes (including those toward phylogenies and disparate morphologies) in the fossil record to confirm and validate the evolutionary tree of ancestry totally common and/or at least represent the major environmental changes that occurred over millions of years, is called the "paradox of morphological stasis" (PMS), and in this work we will demonstrate its relation with the geochronology and stratigraphy under the impact of asteroids occurred in the earth.
RESUMO - A anomalia surpreendentemente encontrada de que não há mudanças significativas morfológicas (incluindo aquelas em direção as filogenias e morfologias díspares) no registro fóssil, para confirmar e convalidar a árvore evolutiva de ancestralidade totalmente comum e/ou pelo menos para representar as grandes mudanças ambientais ocorridas ao longo de milhões de anos, é chamada na literatura de “paradoxo da estase morfológica” (PMS - paradox of morphological stasis) e neste trabalho demonstraremos sua relação com a geocronologia e estratigrafia sob efeitos de impactos de asteroides ocorridos na terra.
For a long time evolutionary biology has proposed several solutions for PMS and associated fossil patterns. In a quick reading of the material data we could see that: 1) Evolutionary mechanisms are absent in the fossil record, at least materially, due to the taxonomic poverty of 250-300,000 species in a sea of estimates of trillions of fossil samples on the planet 2) Technically equal for numbers of body patterns of fossils, when compared to the immense current biodiversity of estimates of 8 to 100 million species, reveal not only that evolution totally stopped in relation to the diversification in the fossil record, as contrasted in accelerated rhythm today, but even so acceleration, occurring with numerical limits of almost same number of fossil body patterns; 3) Morphological stasis also reveals constant and/or segregated environments, where even beings of the same genotype would have to live without changes and any environmental, nutritional pressures, and even without time for foreseeable morphological changes to occur in the course of so long time to maintain such a morphological pattern; 4) Repetition of the same fossil species in distinct strata, as evidence of population burial appearing in distinct strata, and the idea of forms interspersed over millions of years is understated by data demonstrating that virtually all species morphologically change over time (except very rare cases); 5) The emergence of ready-made life forms as standard in the fossil record represents what we call the unprecedented segregating and stratifying catastrophe of species, for if this ready pattern repeats, this suggests, itself, evolutionary perspective and perception, something not happened before. The ready-to-appear pattern tells us something that has buried and fossilized the ready-made beings and refutes the idea of several Cuvier catastrophes current in the various mass extinctions of the theory of modern synthetic evolution, giving rise to a stratifying catastrophe containing repetition of the same species in different strata (SRABURC - Standard of Ready Ancestors Buried in Unprecedented Catastrophism). 6) Ready emergence also has a connection with the controversial subject of "irreducible complexity" that already challenged through thousands of biochemical studies of the cell and life systems, about the impossibility of existence and survival of beings and systems without being ready (Behe , MJ, 1997, 2009; Khun, 2012, Looning, 2005) (and regardless of what this evokes science does not prohibit evoking anything but should stick to the facts). These observations also tell us that 7) a rapid stratification burial scenario of much of the biodiversity, something consistent with rapid, catastrophic and high sedimentary rates (Sadler, 1981) found in the thicknesses, widths, extensions and unique packets of sedimentary and igneous layers , where an increase in burial diversity (fossils) followed by a coordinated decrease in the proportion of fossils could also mirror stratigraphic movements in the transports of marine transgressions and regressions, not necessarily stories of increased diversity followed by mass extinctions. This interpretation of the data would be wholly dependent on the fact that the high-scale geochronology of time in the dating mainly of the sedimentary layers, called "absolute", were totally wrong and that Cuvier's (1769-1832) old idea of faunistic succession and revolution, intercalated for a long time, and its updates in the modern synthetic theory of evolution, in fact, would be only a moment in the geological history where diverse stratifications were being constructed containing repetition of species of the same population. And finally ,
8) we justify the recommendation of new studies related to the possible flaws of conventional geochronology by studying patents of methods of acceleration of radioactive decay, aging rocks in billions of years in only 30 minutes, and behavioral observations of decay accelerating atomic plasmas radioactive, most likely generated on the earth by the fall of large cars.
Keywords: Paradox of Morphological Stasis, Geochronology, Taxonomy, Fossil Statistics, Living Fossils, Ancestral Basic Types, Catastrophism and Neocatastrophism, Real Time Speciation, Rapid Speciation, Morphological Stasis, Punctualism, Saltacionism, Parsimony, Epistemology, Radioactive Decay Acceleration , Uniformism, Spontaneous Segregation and Stratification, Paleontology in T. Impacts, meteor, asteroid, plasma, tokamak, piezoelectric, radioactive decay, uranium.
INTRODUCTION
In scientific research, parsimony is the economic choice of justifications for an observation, thus seeking the simplest and optimized explanation possible. Most of the time, it is considered the best way to judge a hypothesis (Courtney, 2008). We present in this work what we consider to be the most economical and near path to seeing the paradox of morphological stasis (PMS) in the fossil record, "which, besides being something unexpected, is even more dramatic because 'studying morphological conservatism in the long run is difficult in contemporary systems, because few extant animal strains are known to remain morphologically static in relation to geological time scales. (Lavoué et al, 2011). "The same fact is observed by Peter Williamson, a professor of geology at Harvard University, in suggesting that neo-Darwinism has failed to explain the systematic discontinuities in the fossil record:
"The main problem is morphological stasis. A theory is only as good
and conventional neo-Darwinism, which claims to be a comprehensive explanation of the evolutionary process, failed to predict the wide morphological stasis, now recognized as one of the most impressive aspects of the fossil record. "(Williamson, 1981, p. 214).
For a long time in evolutionary biology, the PMS has confronted "evolution-fact" (more experimental and observable) with historical evolution (more conjectural and deduced), where the historical and geochronological part presents one of the major problems of synthetic theory of evolution (Mayr, 2002, Futuyama, 2010, Voge, 2016 and Loenning, 2004). This has led researchers to develop numerous justifications, sometimes extremely complex, sometimes extremely distant from material facts, from concrete data-based paleontology data (Sepkoski, 2013), to try to harmonize the lack of evolution and the lack of morphological changes , in stasis in the fossil record, surprisingly present during supposed immense geochronological periods, what really, by what we know of evolution occurring even in real time, would be a complete absurdity.
Most of the works that face PMS also accumulate new solutions, and others present case study solutions declared as almost "exclusive" or "extraordinary" (Lavoué, 2011), implying that for the rest of living beings, or for non-extraordinary cases, remains unsolved. From observations of great possibility of error in geochronology due to the acceleration of decay caused by plasmas, high geochemical temperatures (Kennett, 2015) and other particle accelerating effects (piezoelectric and sound waves), due to the fall of large cars (Figure 1 ), thus guaranteeing the possibility of an interpretation of the data, directly and without need of justification, due to the exemption of traditional geochronology that imposes a need to justify the evolution occurring even without morphological data for it, and also exempt from the old idea of Cuvier (1769-1832) who remains constant until now in the modern synthetic theory, to explain partial successions of stratigraphic faunas, ignoring that there are already modern substitutive proposals of models of stratigraphic separation in the literature (Minoletti, 2009, Dilly et al., 2015: Berthaut , 1986, 1988, 2002, 2004, 2010, 2011, 2012, 2013, 2014. Lalomov, 2007, 2013; Julien, PY, 1993) that, after analyzing the data, a geochronological and stratigraphic review is necessary and is shown to be a very pertinent alternative to deal with practically all the problems raised by the SMP in a parsimonious, simple, economical way and more communicable with evolutionary, sedimentary facts, sedimentary and stratigraphic consequences of impact catastrophes, and with other anachronistic dating perspectives always requiring calibrations, adjustments and justifying hypotheses.
Acceleration of radioactive decay
There are a number of publications and authors advocating acceleration of radioactive decay (Gentry, 1968-1982, Brown, 2013). And among thousands of citations, we can cite William A. Barker who describes his patent application for acceleration of radioactive decay (as a method of decontamination of radioactive materials), on Dec 31. 1991, as follows:
"In general, the scientific community believes that the decay rate of a radioactive nucleus is unchanged. However, it is possible to change the decay rate by changing the emitter environment .... In this way, the decay rate of radioactivity of the materials is greatly accelerated and the materials are thus decontaminated at a much faster rate than normal.The stimulus can be applied to the radioactive materials by placing those materials within the sphere or terminal of a Van de Graaff generator where they are subjected to the electrical potential of the generator, such as in the range of 50 kilovolts to 500 kilovolts, for at least a period of 30 minutes or more The present invention is based on the fact that the rate of decomposition of radioactive materials can be accelerated or strengthened and thus being controlled by a stimulus, such as an applied electrostatic potential.This potential, for example, is incorporated into the mechanical tunneling equation quantum mechanical coefficient for the transmission coefficient T * T, including an additional potential energy "
Many other papers and patent applications for methods of accelerating radioactive decay and decontamination of materials are described in the literature (An Kinderewitscg, 2003; Gorodezki, 2005). Willian Parker's equipment quoted needed 50-500 kilovolts to generate decay acceleration, how many millions of kilovolts would it take to drop only 1 large car, and what would be the consequences in terms of acceleration of decay and aging rocks?
In addition to the fact that the experiments with nuclear fusion revealed in numerous tests and projects such as tokamak equipment, that, through plasma and temperature systems, can not only increase decay but even alter core of stable elements (Bosch, , And the effects of plasma and other particle accelerators during the fall of large cars (of which we have cataloged (Fig. 1), we can simulate here an interpretation that is exempt from the traditional geochronology due to the fact that it can no longer be (at least about 0.2%), since we have made a significant contribution to the accelerated decay tendency in relation to the diameter of the less "absolute") in the face of such tests and facts and other anachronistic dating perspectives, without therefore needing such innumerable ad hoc justifications, and may simulate an interpreter will with the data as they simply are and are.
The free interpretation of conventional geochronology, traditionally taught over the last two centuries, saves, as it were, a series of juggles justifying anachronistic anomalies that swarm in scientific discoveries, but it will bring new challenges such as if we do not have this time distancing the other, then could we get closer to NASA studies of multiple impacts on Earth's history? (Spray, 1998, Donald R. Lowe, 2004, Bunch, 2012, Witke JH, 2013, Kennett, 2015)
Could the fall of large racing cars age rocks by the acceleration of radioactive decay? In the figure below we notice a clue in this direction:
Figure 1 - Line of increase of age related to the diameter of races. Sodré & Luther, 2017
Image
We observed that the larger the car, the greater age and dozens of small cars assembled around smaller ages. The fall of larger cars represents the potential for greater acceleration of particles and even the appearance of unstable elements (Brown, 2013).
Pattern of Emergence of Ready Forms in the Fossil Record
If the geochronology of billions of years cannot be affirmed with all the alleged absolute certainty, then we would have serious stratigraphic and impact consequences, since we would shorten the sedimentation time and gather more and more impacts closer to each other, approaching from the idea of publications suggesting asteroid rain and multiple impacts on the earth as a possibility to explain what violently smashed the 70 km thick continental crust, and formed most of the extremely catastrophic scenarios on earth, as well as an endless list of events which would require large global magnitudes of events such as coming to produce unimaginable sedimentary rates (Sadler, 1981) that reached the continents and buried alive entire continental giant animals.
On the moon, there are 30,000 crater marks (astroblemes) of large and small asteroids and meteors, and on earth, we have only 200 astroblemes. As the moon is very close, and the earth has a stronger attraction, it is deduced that being its diameter, 3.67 greater than the moon, it has received at least 4 x 30,000 asteroids, that is, more than 100,000 impacts have been eroded, covered up, and/or are yet to be discovered on earth. Or in Zellner's words:
"New high-resolution orbital data from recent lunar missions, resolution improved analytical instrumentation sensitivity, development of new analytical techniques for age acquisition for lunar samples, re-evaluation of literature data, and updated dynamical solar system evolution models that take into account these new observations led to new interpretations of the early bombardment of Moon (and by proxy, the Earth) "(Zellner, September 1, 2017).
The continental crust has an average of 70 km of thickness. In order to break it down, as violent as it was (as we can see on the platform of South America and on the platform of Africa, where there are beneath the sea coast, gigantic eruptions similar to those of the Guanabara basin in Rio de Janeiro-Brazil, which it should also be like the thousands of others underwater, but for some reason still mysterious and unexplained, it is uplifted.The mystery of why only this piece of the platform would be uplifted with a great depression in the center, surrounded by great volcanic eruptions of very high scales suggests an immense catastrophe as the source causing such igneous formation.
The emergence of ready life forms, as standard in the fossil record, communicates with the idea of impacts that would generate large erosions and sedimentation acting along with great marine transgressions and regressions,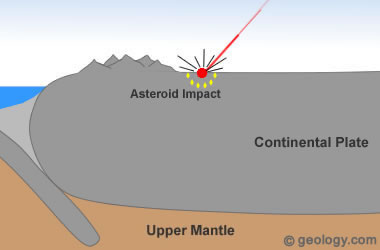
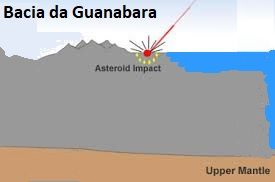


Popigai Crater in northern Siberia, Russia . ttp://geology.com/articles/diamonds-from-coal/ Diagram from Guidebook 27, "Geology of Big Bend Ranch State Park, Texas" by C. D. Henry. Bacia da Guanabara - RJ - Brasil Image CBERS image of the Guanabara Bay (Source: INPE) - http://geologiamarinha.blogspot.com.br/2010/03/ Image The Guanabara Bay from the perspective of the East.
The emergence of ready life forms, as standard in the fossil record, communicates with the idea of impacts that would generate large erosions and sedimentation acting along with great marine transgressions and regressions,deixando rastros de leaving traces of sediment layers especially in the basins of the earth. The patterns of ready emergence can be interpreted as a consequence of gigantic associated catastrophes that occurred in relation to life on earth in an unprecedented way that would have to form several strata containing samples of several ready-made living beings never "evolutionarily" arising in others strata. Studies in this direction of multiple impacts glimpse such depositions and stratifications as:
This pattern discards the presence of previous evolutionary mechanisms for the first life forms, because if one or the other emerged, alternating with evolutionary antecedents and others without antecedents, we could even detach this point and consider it accidental due to a series of justifications (erosions, evolutionary jumps, etc.), but these would generate stochastic factors rather than standard regularities in the strata, and the fact that this event is standard imposes on us an immense burden of having to admit that ready-made forms simply happened on earth and have no support at all. less material, data, fossils, to say that they were evolved, or that stochastic fossil erosions and rarity would form this pattern in various strata, etc. The option to understand that these ready forms would represent an immense catastrophe for the first time buried all life on earth, communicates with what we can call "the standard of ready ancestors buried in various strata in a new and recent catastrophe" (SRABVSURC) of ready ancestors buried in various strata in unprecedented and recent catastrophe catastrophism). Not to mention the fact that thousands of scientists most closely linked to cell biochemistry make living systems in general need to be ready because they require "irreducible complexity" (Behe, MJ, 1997, 2009; , 2005) in order to exist, adapt, survive and evolve.
Dr. Kjetil L. Voge sent us his recent publication in which a broad bibliographic review of the problem of SMP is made (Voge, 2016). Numerous related works start to touch the problem presenting some solution to the surprising anomaly of morphological stasis occurring in calculated Ma (millions of years). All seem not to doubt or question with respect to the problems of "untouchable" geochronology and their dating "Absolute" nor of the conventional stratigraphy, but seek within these models, to create justifications of the most diverse for the PMS.
But this is not a rare case where geochronology and stratigraphy impose justifications for anachronistic data, we observe that many other points of paleontology go through the same situation, from the millennial wisdom of the Indians when pronouncing the word "itarare" (soft water in hard stone both beats until it bores ") where we see trillions of rocks being impacted without suffering compatible erosions for a long time, to points that will be even more frontally emphasizing anachronism, such as the dating of soft tissues stiffened with browned hemoglobin lees dated in extraordinary 60 to 120 million years.A complete scientific absurdity that prefers to justify and speculate on mysterious superpowers of protein and soft tissues to preserve for so long, than to question date absolutism.the absurdities are so many that for this article not to turn into a book, we have chosen only three rather flagrant examples, in Table 2 below, citing a very small proportional sample of related articles, all of them seeking to find "ad hoc" outputs for surprising and anachronistic anomalies. I would like to point out that some of these articles seem to mock the problem so that they can be published, without directly confronting the conventional paradigm, or, analyzing Dr. Lavoué's words, we are even perplexed by statements and confessions as imploring solutions such as long-term morphological conservatism is difficult in contemporary systems because few extant animal strains are known to remain morphologically static in relation to geological timescales. " (Lavoué et al, 2011).
From the scandal of Eldredge and Gould's punctuality, it seems that the academy represented by more attached evolutionists tries to justify as much as possible the current geochronological system, which is gradually undermined by increasingly ironic questions, bordering on the forcefulness and more and more open, without breaking completely with the paradigm hymen. There is a group of PMS justifying publications that appeal, among other justificatory ways, to "stabilizing selection", while accumulating other solutions indirectly or sometimes directly confessing (Voge, 2016) its insufficiency, since only a great catastrophe such as (Sadler, 1981) and we would have to have non-stabilized morphologies along with stabilized ones, demanding extraordinary luck for those who defend this thesis, so that the same catastrophe did not bury those not stabilized by the selection natural, unless natural selection would act together with all species to form a pattern of stability. Note that the selection, which should be selecting at least a few "disparate" to be directed toward forming the evolutionary tree of all-common ancestry in the fossil record, is now, in the face of the PMS, highlighting and giving greater prominence withinseveral contingent features of natural selection, its conservative characteristic of stabilizing forms (conservatism that in other circumstances and questioning is even avoided). The arguments and stratagems of highlighting true points, but sometimes in isolation, multiply in computational models, floating rates, and repeated confession that such justifications are still insufficient (see Table 2). Note that the selection, which once needed to select "disparate" to be directed toward forming the evolutionary tree of all-common ancestry in the fossil record, is now, as a joker, facing the PMS, being read and highlighting its power conservative way of stabilizing forms. In the literature review of Voge it is summarized as follows:
"The assertion that directional change was rarely observed in the fossil record and that stasis was the dominant mode in evolutionary lineages (Eldredge and Gould, 1972, Gould and Eldredge 1977, Cheetham 1987, Jackson and Cheetham 1999) provoked intense debate among evolutionary biologists (Gould 1980, Charlesworth et al., 1982), which is still ongoing (eg Lieberman and Eldredge 2014, Pennell et al., 2014a, b, Venditti and Pagel 2014) Part of the legacy of equilibrium theory (Gred and Eldredge 1977, Gould 2002) is the finding that established species generally show a minimal net evolution over time in the fossil record.It was also found that the differences between species in the fossil record are less than expected due to genetic drift alone (eg, Lande 1976, Lynch 1990, Cheetham et al., 1993) .The domain of slow evolution in the fossil record is not directly derived from what they know those of microevolutionary studies: quantitative traits generally present substantial genetic variation (Houle 1992, 1998; Hansen et al., 2011), a strong selection is common (Hereford et al., 2004), and a considerable amount of evolution is frequently observed at time intervals from years to decades (Hendry and Kinnison 1999, Kinnison and Hendry 2001, Hendry et al., 2008, but see Merila et al., 2001). The contrasting observations of evolutionary change in long and short time scales are known as the "stasis paradox" (Wake et al., 1983, Hansen and Houle 2004; Futuyma 2010) and makes the long-term history of Life seems almost decoupled from the evolutionary process we study at shorter time scales. The assertion that directional change was rarely observed in the fossil record and that stasis was the dominant mode in evolutionary strains (Eldredge and Gould, 1972, Gould and Eldredge 1977, Cheetham 1987, Jackson and Cheetham, 1999) provoked an intense debate between (Gould 1980, Charlesworth et al., 1982), which is still in progress (eg, Lieberman and Eldredge 2014, Pennell et al., 2014a, b, Venditti and Pagel 2014).
Image
The present study will exempt the cause of these ad hoc explanations: What we judge to be the submission itself and the non-questioning to the uniformitarianism of the constancy of radioactive decay, which assures us that like rats trapped in the trap, we can only eat the cheese and justify because evolution has come to a halt in half a billion years (if we only consider the fossils of the Phanerozoic, because if we were to talk about pre-Cambrian bacteria and fossils we would have to assume morphological stasis in 3.5 billion years). Or as we can see in this table that represents only a small sample of this dependence
Image
Definitions of Species, Gender and Families, and MPTG
For decades, evolutionary biology, in the face of the "plasticity of living beings" since Spencer, 1820-1903 (Lightman & Bernard, 2016), is so prominent, especially since Charles Darwin's immense bibliographical revision, have difficulty understanding and defining the meaning of "species". Currently, there are more than twenty-two different species concepts (de Queiroz, 2005). The use of different concepts leads to mismatched comparisons in science that aims to systematize and organize knowledge. On the other hand, catastrophic biologists have for decades used dynamic and plastic species more appropriate and flexible terms and concepts such as "type" or "group" for what they consider categories of genetically related organisms that, in tests of artificial crosses, chromosomal pairing and at least the onset of embryo with paternal and maternal characteristics (Junker & Scherer, 1996). Thus, it becomes practical to group clades around these compatibility tests in the crossover.
Each of the various categories of species, subspecies and varieties we see today were designed to diversify from a common ancestral common fossil type, so we will consider in this work, the greatness MPTG (morphological patterns around the taxon genus), morphological patterns around genres . This greatness is identified with ancestral fossils, with the falsifiable hypothesis of the basic types (Marsh, 1941), since "new forms become more and more refinements of existing forms" (Benton et al, 2007). Among other observations, we can say that the morphological disparity in the fossil record, when we compare higher taxa, is high, and the diversity of species in the fossil record is low. That is, a true mirror of basic ancestors of which most of us (as biodiversity) descend. Similarly, many trunks and shrubs with thick branches (disparity), in the fossil record versus shrubs with endless foliage and twigs today (diversity). This does not cancel out morphological variations in the fossil record, of course, they exist within a morphospace that houses even the same genotypes, and it is even expected that they exist evolutionarily, since the mother species have more and more, bigger gene pool, since they were not yet (Mayr), or by the accumulation of deleterious genes that bequeathe the engine of genetic entropy (Sanford, 2005; Crabtree, 2010).
Several examples of disparity and diversity are cited in the literature (Benedict et al, 2016). That is, we defend that MPTG basic ancestors in their characteristics of morphological (and low diversity) disparities were buried, usually catastrophically and with "sudden death", to become fossils, and this model tells us a story of a one-moment period (Benton et al, 2007; Futuyama, 2010). 2) Permanence with a high number of species, such as: (1) lack of rapid or slow speciation, which, if it were to exist, would enrich the fossil taxonomy, species, which would require a stable environment (fossil replication without environmental pressures that would provoke adaptive and evolutionary manifestations), 3) a living population's disastrous disaster evidenced by the repetition of the same fossil species (which decharacterizes punctuality, long time, and would replace partial faunistic succession for "segregation and spontaneous stratification" SEE (Minoletti, 2009, Berthaut, 1986 , 1988) that explain the presence of several different species segregated in the fossil record, as well as do not need to appeal to "ad hoc" to explain polyster trees and thousands of anachronisms 5) Fossils of immense vertebrates and (6) Drastic change in the environment by generating the adaptive radiation of the species in recent layers in the millions of species of the present biodiversity diversified by more than maintain a similar number of corporeal patterns (Wise, 2013).
Many species and their variations, observable today, reflect the same morphological patterns around the genus taxon (MPTG), although much of the literature designates the upper taxon families as morphological patterns, which results in the fact that we read in the literature "extinction of families "(which may actually be just genres). On this problem, after consulting various experts and literature requesting global data on species quantity, genera and families in each geological period, and receiving unresolved laconic responses, let us begin to understand statements such as: "There is currently no agreed definition of disparity , much less any consensus on how to measure "(Wills et al, 1994), so I chose as the best synthesis the e-mail response in 2013, led by Stephen Jay Gould's Stephen Jay Gould, Dr. Kurt Wise, the which practically repeats (but with greater richness) the same observation of specialists consulted, that the definitions and organizations, due to having numerous criteria, are missing:
"The best early estimates (by Dave Raup and Jack Sepkoski in the 1980s) were based on a family-level fossil record (namely, Sepkoski's family-level compilation of the marine fossil record). From the number of families that went extinct, the current intra-family species diversity was assumed to be true of the fossil families, and the necessary% extinction was calculated that would result in that much family extinction (by computer bootstrapping, etc.) . Later, after Jack Sepkoski had compiled genus-level data for the marine fossil record (2002, Bulletins of American Paleontology 363), the same sort of calculations were done with the genera that had earlier been done with families. Not only have the species-level data not yet been compiled, but many paleontologists consider that data would be unreliable (because of the differences of opinion on how to define paleontological species). "
Since the classification at the family level is very contradictory when we understand that the family grouped genera that were not extinct, it is common to read in the literature data referring to one thing and another at the same time, or on percentages occurring in the mass extinction without the previous number of where these percentages were withdrawn. However much it has not generated so many corrections in describing extinctions (interpretation) or fossil diminution in the stratigraphy (fact), (Sepkoski, 1993), this makes it impossible or difficult for other perceptions especially of evolutionary relation in the paleontological study.
Recently, non-extinct bivalve genera were classified as families (Gibson, 1996). And because of this, we try to understand MPTG as a flexible way of dealing with these inaccuracies, which can help us in organizing the general understanding of these classificatory difficulties.
Therefore, MPTG is what we consider as the peculiar characteristics that range from the basic types of fossil matrices to their descending diversification in the present, which still exists today, except extinct families and "genera" present in most of the various species and their variations found in nature (Eldredge and Stanley, 1984). Many living species today, and their variations reflect similar morphological patterns. The permanence of these same fossil morphological patterns, however much the number of species increases, makes it easier to perceive the limits of the evolution around MPTG, locating and orienting the possible changes to be registered in the fossil record and tested their kinship today (Junker & Scherer, 1996). Also, the fact that this discontinuity is even more marked in the fossil record, the higher the taxa, strengthens this idea of systematic discontinuity (Carroll, R. L., 1992).
The thesis of the basic or ancestral types (Marsh, 1941) has been supported by the fact that the fossil record presents low variability (morphological stasis) and low special radiation among fossil species (Zimmerman, 1960; Martens, 1997; Albrecht and Wilke, 2008), regardless of their respective phenotypic or genotypic plasticity or malformations (Ghalambor et al., 2015). In addition, other factors corroborating the thesis of the ancestral basic types are the more than 4229 well-documented genera of "living fossils" that are so called because they have undergone few changes over time, thus remaining similar to those found in the registry fossil (Romer, 1966; Whitmore, 2013a; Whitmore, 2013b). Probably reflecting ideas of his day, Darwin came to quote this current deduction by writing:
"Is not there a real greatness in this way of considering life, with its various powers originally attributed by the Creator to a small number of forms, or even to one?" Now while our planet, obeying the fixed law of gravitation, continues to revolve in its orbit, an infinite number of beautiful and admirable forms, coming out of such a simple beginning, have not ceased to develop and develop yet! ... The venerable and Reverend W. Herbert, later dean of Manchester, wrote in 1822 in the volume of the Horticultural Transactions, and in his work the Amaryllidacées (1837, pp. 19, 339), that horticultural experiments have established, without possible refutation, that botanical species are not more than a higher class of more permanent varieties. "It applies the same opinion to the animals and sees that the unique species of each genus were created in a very plastic primitive state, and that these types subsequently produced, mainly by crossing and also by variation, all our existing species. In the Nouvelle Flore de l'Amérique Du Nord (1836, p. 6), Rafinesque expressed himself thus: 'All species could be once varieties, and many varieties gradually became species, acquiring permanent and particular characters'; and a little further on (page 18) he adds: 'Except for the primitive or ancestral types of the genus' "(Darwin, 1866, Chapter 9, pp. 25-26, 577, our emphasis).
When we speak of morphological stasis in the fossil record, we refer to the sedimentary layers between Ediacara / Cambrian until near the Pleistocene layers, or superficial layers, as evidenced by several publications based on the theory of punctuated equilibrium (punctualism) proposed by paleontologists Niles Eldredge and Stephen Gould (Levinton and Chris, 1980, Woodruff 1980, Williamson 1981, Eldredge 1986, Van Bocxlaer and Hunt, 2013). And not in the current layers where the variation radiates in multiform variations and fossilization also occurs.
The morphological patterns associated with the MPTG genus can be observed in the fossil record appearing suddenly in all sedimentary layers, with an expressive appearance in the Cambrian and abrupt emergencies with no gradualism in every geological column, but a surprising repetition. This finding has been reported for decades by the adepts of punctuality who still admit to morphological stasis and radiation blooming in speciations only in the most current geological strata (Levinton and Chris 1980, Woodruff 1980, Williamson 1981, Eldredge 1986, Van Bocxlaer and Hunt, 2013). A recent evolutionary study corroborates this assertion by saying that:
"The dominant view of evolution based on the fossil record is that established species remain more or less unchanged during their existence. On the other hand, substantial evolution is routinely reported in contemporary populations, and most quantitative traits show a high potential for evolution "These contrasting observations on long and short time scales are often referred to as the stasis paradox, which is based on the fundamental assumption that periods of morphological stasis in the fossil record represent a minimal evolutionary change. (Voje, 2016).
Thus, the present article has as object to study these observed and well documented facts: sudden fossil emergence, morphological stasis, repetition of the same fossil species in contrast to the explosion of adaptive radiation revealing all potential of plasticity and malleability of phenotypes and genotypes of beings which should be present in the fossil record if history had occurred there, since "morphological diversity decreases along with taxonomic diversity. This pattern suggests heterogeneities such as high extinction and / or reduced origin in certain regions of the morphospace "(Foote, 1993)
The speciations and variations that, as we have seen, carry the MPTG can be observed occurring in real time and historical-archaeological, but are absent in the same proportion, in the fossil record. This becomes relevant insofar as we perceive the reproductive success of the variations and their permanence over time, since the variation around gender does not require large bio-transformations, but must occur in any way, around the genus (MPTG) in fossil samples. Today, surprisingly, according to paleontologist fossil scientist Dr. Kurt Wise, technically, the number of bodily planes in the fossil record does not exceed the number of body planes today (Wise KP, 1989), and according to this statement, we have almost the same number of body patterns between the 250-300,000 fossils cataloged, compared to 2 million living species now cataloged, with estimates of 8.7 million and estimates that go up to 100 million species today) and the tiny 300 thousand species of the Cambrian / edited to the pleistocene. (Woodmorappe, 2000, Sadava et al., 2009, Mora et al., 2011, Catalog of Life, 2016). When comparing morphological patterns, one would expect that we would have a much larger number of PMTGs in the fossil record if it mirrored a 544-million-year bio-diversity sample corresponding to the Phanerozoic. Therefore, the fact that the present number of species, in the millions, can not transpose the number of body patterns present in the fossil record, suggests a delimited evolution to morphological patterns or ancestral basic types (baraminology) or MPTG.
Real-Time Speciation
The surprise of many in the face of rapid speciation and significant morphological changes in just one generation reflected the theory's lack of prediction of what one would expect in terms of time.
We understand real-time speciation as a phenomenon in which two or more populations of the same species change in new arrangements of preexisting genetic information, caused by separation by geographic barriers and deaths, but in a timely manner so that observation of the entire process from start to finish. (Furness et al 2015). Empirical experiments promoting real-time speciation have been tested successfully and increasingly challenging the PMS, (Ghalambor et al, 2015), which demonstrates that "no organism ignores its environment" (Ezard et al., 2016 )
We can also classify the process of speciation in two ways: in real time or in historical-archaeological time. Real-time speciation is one in which there are limited modifications to the basic type, equivalent to the rapid emergence of a new "species", observed ̶ by experimentation or uncontrolled observation ̶ within 50 years. Speciation in historical time, in turn, refers to bio-modifications equivalent to a new "species" over 50 years that may or may not be observed due to the lifetime of the observer or research project. In these cases, there is a 50% chance of observation and the remaining 50% depend on the use of the deduction, for example, from chromosome analysis and calculations of radiation rates (Trivedi, 2000).
The role of genetic drift in the process of speciation
There are several mechanisms involved in the process of speciation (epigenetics, genetic drift, natural selection, environmental and geomagnetic influences in the crossover (Gorelick, 2005)). Because speciation is closely related to genetic drift and the consequent loss of gene pool from an earlier population, studies of the many basic ancestor model (SRABUC) that rely on real-time speciation become critical in explaining current evidence of limits to adaptation in different living organisms (Bell, 2013).
Genetic drift is a mechanism that randomly and abruptly modifies - due to catastrophes or to diverse insulation, for example, - the allelic frequencies of a population (Ridley, 2006; Freeman and Herron, 2009). On the other hand, it is believed that natural selection is directed, that is, it eliminates many deleterious mutations, necessary mutations if lost together, ignores the neutral and deleterious mutations (especially those that manifest after the reproductive period), select the surviving traits and / or advantageous pre-existing or communicating in the face of epigenetic response to the environment (Sanford, 2014). However, as the American biologist Lynn Margulis put it, "natural selection eliminates, and perhaps maintains, but it does not create" (Teresi, 2011, 68). In addition, due to the fact that genetic drift does not distinguish between good and bad genes, and natural selection also fails in its filter, this results in loss of genetic variation (Lacy, 1987), leaving species genetically poorer and "increasingly closer to extinction. " In other words, drift cuts off and impoverishes the population of its "gene pool," and natural selection less what was left.
In this sentence, I will make a modification so that it becomes more technical. It is known that the effect of the drift is inversely proportional to the size of the population, being able to appear in different moments of the history of the species and the own humanity. In this sense, it is possible that drift played a key and overriding role to that of natural selection in the process of rapid speciation after a major catastrophe.
The Speciation Process After Catastrophes
Great world catastrophes are the formators of most of the sedimentary layers present on the globe, which is associated with immense floods and destruction throughout the globe (Souza Jr, 2008). The consequences of mass mortality, endogamic stress on surviving species and geographic isolation, gave, in addition to other factors, very favorable conditions for rapid speciation (Wilmer et al., 2011). The SRABVSURC model provides for the burial of biodiversity around the world by spontaneous stratification in marine transgressions and regressions (Berthault, 1986, Berthault 1988, Brand and Tang 1991, Snelling 1997, Berthault 1998, Chadwick and Spencer, 2006, Berthault, 2013 ), followed by rapid specifications for adaptive evolutionary engines that would diversify the patterns of ready-made ancestors buried in unprecedented catastrophe (SRABURC).
The SRABVSURC model of the short history of the formation of sedimentary layers predicts a time when the few humans and animals surviving and isolated by these associated disasters (of energy and global magnitude) would have begun to repopulate the land, and successive drifts and endogamous stresses of crosses (Prüfer et al., 2013) and on the foundations of ethnicities of similar biotypes among themselves inhabiting fossils of dissimilar halogens such as, for example, miozoids in the Americas on top of fossils dissidents in Lagoa Santa-MG, São Paulo and Mexico. A fact also observed in China of Mongoloids inhabiting Caucasian and Negroid fossils (Ke, Y, 2001) both in human ethnicities and in thousands of other living beings similar to each other isolated from their relatives.
... "Negroid and Australoid, returning to raise a great controversy that calls into question the models of prehistoric settlement of America. Reinforcing the possibility of being the population of Lagoa Santa, like other American populations of more than 7 thousand years, originating from a very old migration of non mongolized groups, these new findings brought back and with force to the scientific scene, a polemic of 160 years. "(Mendonça de Souza, 2006)
In this context, real-time speciation legitimizes the model of a recent evolutionary leap with two bio-differentiating peaks related to the episodes of the onset of human genetic entropy (Fu et al., 2013) after the associated disasters that would impoverish the pool and would give the survivors, by virtue of migration and geographic isolation, the legacy of founding effect - a frequent situation in peripatric speciation - on a planet with a fully reconfigured ecosystem where the epigenetic motor would act to match the needs of the new environment (Ridley, 2006; Eakin, 2014; Weyrich et al., 2016).
Peripaterial speciation is a mechanism by which we can account for the enormous increase in post-disaster diversification. It is a kind of speciation by which new species are formed in isolated peripheral populations (Ridley, 2006). In peripacic speciation, drastically reduced populations make complete speciation the most likely outcome of geographic isolation, because genetic drift acts more rapidly in small populations. Genetic drift coupled with strong selective pressures would cause rapid genetic change in the small descending population (Wilmer et al., 2011).
Current observations may serve as an example for better understanding cases of peripatric speciation. "An analysis of more than 2,000 bird species provides an insight into how the various beak forms of animals evolved and points to a unique rare event as a trigger for rapid early divergence of avian lineages." (Bhullar, 2017).
All this succession of facts can be glimpsed through a scenario where most of the organisms destroyed by these major associated and consequent catastrophes would leave small populations of survivors (Wilmer et al., 2011). According to a report published by Folha de São Paulo about this finding,
"What seemed to fascinate most other biologists, however, is the great speed with which the phenomenon of character displacement occurred. "I believed it would take a lot longer," biologist David Pfening, of the University of North Carolina, told Science. The average reduction of 5% in ta which act in these limited modifications to the basic type, such as epigenetics and energy and temperature aspects acting in the crossover (Fondon and Garner, 2004; Eakin, 2014). According to Dr. Jean K. Lightner, 45 there appear to be three sources for the variations associated with adaptive radiation: 1) hybridization, 2) mutation and 3) environmental screening of ancestral alleles (by natural selection and meiotic drift) (Lightner, 2016).
These observations, if they do not add to the debt of lost links in punctual paleontology, greatly increase the debt of fossil taxonomic variability to the historical geological model that assumes at least five major catastrophes separated by millions of years in the history of the earth.
Interestingly, research linked to real-time speciation both confirms Darwin's observations in functional biology (punctuated equilibrium) and completely destroys evolutionary postulates in terms of geological periods (phyletic gradualism) and is perfectly communicable to models catastrophists who bet on rapid speciation driven by the effect of bio-modifications limited to the basic type.
Baraminology and the study of "basic types"
The sudden and ready appearance of life forms and irreducibly complex systems (Lonning, 2005), as well as the technically equal number (Wise, 1989) of morphological patterns when we compare fossil taxonomy of 250-300 thousand species with estimates of 9 to 100 million suggests the idea of a phenomenon of ready-made ancestral basic types and the numerical permanence of limited evolutionary patterns around them (MPTG). There is a very fluid communication between biological Darwinism and the Biological Darwinism model, where many rely on evolutionary discoveries, such as real-time speciation, to get closer to the historicity of a short period of time, and using the recent radiation as a justification for the whole biodiversity. The theory of synthetic evolution, more specifically acting in the biological field, which teaches the differentiation of species, in this context, has low discord among the most advanced classes of SRABUC, especially in the scientists of the "Baraminology" movement (Marsh, 1941; Wise, Wood and Cavanaugh, 2001, Cavanaugh and Wood, 2002, Wood, 2010, Aaron, 2014). Their disagreements focus more on the scope of the geo-paleontological history between the Cambrian / Edicara and Pleistocene and/or surface layers.
Since 1941, the SRABVSUR model has countered the idea that speciation is synonymous with "evolution" by means of studies related to specifications in the field of Baraminology (Marsh, 1941, Wise 1992, Robinson and Cavanaugh 1998, Jerlström, Wood and Cavanaugh, 2001, Cavanaugh and Wood, 2002, Wood, 2010, Aaron, 2014). US biologist Dr. Frank L. Marsh, one of the founders of the Creation Research Society who coined the word "baramin." (Marsh, 1941; Frair, 2000). It was derived from the combination of two Hebrew words - bara (created) and min (type) - referring to basic types created (species, in Bible versions in Portuguese).
In 1990, catastrophic paleontologist Kurt Wise noted the need for a biosystematics SRABVSURC - a method of studying, naming, and classifying baramin (Wise, 1990; Frair, 2000) or MPTG ancestors as we have argued here. The scientific field has been officially termed "baraminology", which in a simplistic way means the study of the baramins or the basic ancestral types. According to researchers Reinhard Junker and Siegfried Scherer, "basic types are a classification unit, a taxon, the result of systematic discontinuity work as observed in nature" (1996, p. 34; Wood et al., 2003). Put simply, basic types created variabilized over time to what we know today as subspecies.
There are some falsifiable rules for considering a group of species as belonging to a common ancestral basic type. Dr. Junker and Dr. Siegrifield Scherer highlight in the 6th German edition of the book Evolution, a critical textbook:
"All individuals who are linked directly or indirectly by crosses are considered to belong to a basic type (genetic level). And all biological species that clearly resemble one another belong to a genus (morphological level). And all the biological species that in principle can cross each other belong to a basic type (morpho-genetic level) "(Junker and Scherer, 1996, p. 34).
The authors further add that "two individuals belong to the same basic type when the embryogenesis of a hybrid goes beyond the maternal stage of development and contains a coordinated expression and paternal and maternal morphogenetic genes" (Junker and Scherer, 1996, p.34). In addition, baraminologists use a series of methodological adherence evolutionary behavior of populations.
Baraminology, also known as discontinuity systematics, is fast becoming one of the most active areas of SRABVSURC research (Scherer, 1993), and some of its methodologies have been applied and tested even by researchers linked to the geological gradualist model of ancestry common with highlights in peer-reviewed journals (Senter, 2010; Wood, 2011). As we have seen, its main purpose is to determine which organisms share a common ancestor (Frair, 2000). The basic idea defended in this field of research is that there are limits on the possibilities of crossing that cannot be crossed. In this context, baraminologists aim to find "discontinuities" in life history, or the limits of common ancestry (Remine, 1993).
This field of research gains further stimulus from current evidence showing that "specialists" have erroneously classified some species within a given genre because of the "desire" to discover the universal common ancestor (Lopes, 2015). Paleontologists claim that a third of the "species" recognized as being dinosaurs may not even have existed (Horner and Goodwin, 2009). For them, these "species" may not be separate species, but juvenile or subadult stages in development, erroneously identified as being exemplars of other species. In an article published in the journal Science, for example, Schwartz and Tattersall argue that this miracle of the multiplication of species nomenclature has gone too far (Schwartz and Tattersall, 2015).
It is worth remembering that although Baraminology has achieved promising results, its conclusions are not definitive (Wilson, 2010). Because it is a recent field, more research is needed and its newly developed methods and techniques should be better scrutinized in order to legitimize its function and utility in the science toolbox.
Genetic Entropy in Speciation
And what about the costs incurred when speciation actually occurs? Another major obstacle to the gradualist model is that it is neutral in relation to the improvement or worsening of the speciation process, although it assumes that genetic drift causes loss of genetic variation in small populations. The model of many basic ancestors buried in a recent catastrophism (SRABUC), in turn, with the proposal of genetic entropy, argues that the speciation results in the loss of genetic information and the consequent degeneration of the genome due to the adaptations as evasive, (Ariza, 2007, Sanford, 2014, Crabtree, 2013a and Crabtree, 2013b). Further evidence corroborates the SRABVSURC model in order to suggest that this loss of genetic information due to deleterious mutations in humans has recently occurred between 5,000 and 10,000 years ago (Fu, 2013).
The entropy in genetic information is increasingly evident in everything that is observed: genetic drift, selection/elimination, mutations, exhaustive immunological complexities, among other mechanisms. In humans, for example, current estimates are that 100-200 new mutations occur per individual per generation (Nachman and Crowell, 2000; Dolgin, 2009; Lynch, 2010). From these, data range from 1-15% of deleterious mutations that would cause the direct loss of genetic information in humans at each generation (Nachman and Crowell, 2000; Lynch, 2010; Eyre-Walker and Keightley, 1999; Shabalina et al. 2001; Keightley, 2012).
In relation to fitness, a study published in 1997 estimated that the loss of human aptitude, that is, a high genetic cost, which causes humanity to degenerate with each generation due to the depletion of the adaptive resources used for genetic variety (Crow, 1997). In 2010, in turn, another study estimated that human aptitude is declining by 3-5% per generation (Lynch, 2010). Also, another study simulating numerically the accumulation of deleterious mutations, besides showing that most mutations are on the left, (-0.001), that is, tendency to be deleterious, "bad mutations"), they are also accumulating, in a zone "non-selection zone" because they are "quasi-neutral", these mutations have accumulated over the generations causing dozens of "spelling mistakes" in the population genome. (Sanford et al 2008)
Figure 1 - Simulated distribution of accumulation and frequency of deleterious mutations over generations and their effects on population fitness.
Graph in red shows natural distribution of mutations where no selection occurs. The green columns show the current mutations that are accumulating, that is, dominant. Other columns are smaller, recessive mutations that have been accumulating as they reach the near-neutral zone, close to 0.0.
The Dutch zoologist Duyvene de Wit perfectly described this process of genetic impoverishment by stating that:
"When a marginal population clears the way for a new habitat, it can not carry with it all the genes of the maternal population, but only part of them. Each new breed or species that originates from another has, therefore, a poorer gene pool. Consequently, the loss of substance from the gene pool is the price each race or species has to pay for the privilege of existing. If the process of speciation occurs again and again sequentially, there will eventually appear species whose gene pools are so completely depleted that insignificant changes in environmental conditions are enough to extinguish them. Efforts to adapt to environmental changes as a result of insufficient recombination lead ultimately to a minimal genetic state. If this minimum limit is exceeded, there will be no further possibility of survival. For this reason, the tragic and irrevocable fate of highly adapted species or specialized breeds is genetic death "(Kahle, 1999, p.87; Junker and Scherer, 1996).
Therefore, the SRABVSURC proposal is reasonable and is increasingly supported by scientific data when it states that living beings of the past were healthier and more fit than today, without the defects resulting from forced adaptations and distinct selective pressures that they would survive. It is worth remembering that subspecies, the more verifiable in real time, the more they prove bio-modification in a short period of time, as predicted by the catastrophic model.
Fossil record and absence of species diversification
Speciation proportional to the biodiferenciator behavior observed is absent because there are only about 300,000 fossil types repeating from the estimates of trillions or innumerable samples, in all geological strata up to the Pleistocene (Woodmorappe, 2000; Sadava et al., 2009; Loceye Lennon, 2016). This fact indicates the absence of sequences during the speciation process when contrasted with estimates of 8.7 million species living today (Mora et al., 2011).
In other words, the absence of "diversity" in the fossil record reveals that differentiations occur more in the present (millions of species) than in every geological column (hundreds of thousands). This episode indicates a time when living beings did not need to sub-spice (bio-modify) and often adapt to survive because they lived in a more conducive environment for life.
Recent evidence points to the fact that the beginning of the diversification of some genera of plants considered "living fossils", for example, occurred at the same time around the world and in a much more recent period than was supposed, revealing conflicts in the gradualist proposal of the species ( Nagalingum et al., 2011). In addition, it was reported that this rapid diversification was caused by a major climate change. Another similar example comes from Ernest Mayr:
"In evolutionary biology we have species like horseshoe crabs. The horseshoe crab goes back in the fossil record over two hundred million years without any major changes. They obviously have a very invariant genome type, right? Wrong, they do not. Study the genotype of a series of horseshoe crabs and you'll find there's a great deal of genetic variation. How come, in spite of all this genetic variation, they have not changed at all in one hundred million years while other members of their ecosystem in which they were living two hundred million years ago they are either extinct or have developed into something totally different? Why did not horseshoe crabs change? That's the kind of question that completely stumps us at the present time. "
Incidentally, there are several integrated adaptive and modifying engines currently recognized. The fact that they exist in living beings allows us to suggest that bio-modifications never cease to exist.
Another pattern of the fossil record seems to suggest more the movement of segregation and spontaneous stratification than (Dilly et al, 2015: Berthaut, 1986, 1988, 2002, 2004, 2010, 2011, 2012, 2013, 2014, Lalomov, 2007, 2013, Julien , PY, 1993), reproduce in the laboratory. One of them is the coordinated disappearance:
"Coordinated disappearances. A large number of fossil species may disappear from the geological record at a specific stratigraphic level (see Figure 3). The disappearance is never complete, but there are several examples where estimates indicate that more than 50% of species disappear on the same stratigraphic level. The boundaries between stratigraphic levels are often identified on the basis of coordinated disappearances. The greatest example of this is the disappearance of nearly half of the families (see Figure 3) and about 95% of all species at the top of the Paleozoic. Dinosaurs and many other groups of reptiles and marine invertebrates disappear from the record at the top of the Mesozoic. Other examples of large-scale coordinated disappearances occur in the upper Ordovician, near the top of the Devonian and the top of the Triassic. Coordinated disappearance is a pattern of subtraction. No sustained trends have been reported in this pattern. "(Gibson, 1996)
Figure 2 - Stratigraphic pattern of the number of families of marine invertebrates represented by fossils at each stratigraphic level. (After Sepkoski 1993, see Note 6.)
Stratigraphic Level: V = Vendian; Cm = Cambrian; O = Juridical; S = Silurian; D = Devonian; C = Carboniferous; P = Permian; Tr = Triassic; J = Jurassic; K = Cretaceous; T = Tertiary. Points marked 1-5 represent the five largest "mass extinctions"
The spontaneous stratigraphic segregation of bodies and common sediments explains a certain alternation between the presence of many fossils of a category followed by lack of it (which is interpreted as mass extinction).
PMS generates a great deal of need for deductions and models of mathematical models, macroevolutive simulations in computers, among innumerable justifications, which as Dr Tamborini referred to in relation to idiographic paleontology, are "scientific in a purely descriptive sense, but are completely useless" for biological investigations. "(Tamborini, 2015).
Allegations that, in 90% of the total phanerozoic of 544 million years, bio-modifications have stagnated, repeating in the geological column the same species, and only recently in the higher layers has there been large-scale (radiation) Gould, 1981) give an example of scientific descriptions having greater weight than the data itself. In addition to these justifications for PMS, we have a huge list of ad hoc justifications for clear geochronological anachronisms such as soft tissues and the presumed preservation of proteins from fossilized animals dating to 60-120 Ma; where the authors, instead of questioning the untouchable absolute dating, seek to find ways to justify incredible preservations of fragile proteins, another example comes from the own dating radiometry, with the unexpected presence of carbon 14 available in "uncontaminable" 300 Ma diamonds (Baumgardner, 2005), and almost a hundred dating perspectives and methods, which are forgotten, by the preference of what combines with the time of the current geochronological paradigm. See TABLE 2.
Figure 3 - Flow of geochronological interpretations regarding PMS, soft tissues, carbon 14, where the assumed geochronology forces researchers to make ad hoc justifications to the right of interpreting the fact itself.
It is estimated that C14 in a sample has totally disappeared after 100-250 thousand years, since its half-life is only 5730 years, a fact that leads many to the dogma that it does not test things supposedly of Ma with C14. However, challenging this convention, thousands of tests were made by geophysicist Dr. John Baumgardner's team at the Los Alamos, Texas, laboratory, which demonstrated that 300-500 million-year-old rocks contained quantifications of C14 dating content; before ad hocs justifying such anomaly, critics resorted to contamination, so they tested even on "uncontaminable" diamonds (the possibility of contamination in diamonds becomes negligible) by giving similar results and confronting conventional geochronology. (Baumgardner, 2005)
Dr. Tom Kemp, curator of zoological collections at the University of Oxford Natural History Museum, made the following admission: "As is now well known, most fossil species appear instantly in the fossil record, persist for a few million almost unchanged years, and only disappear abruptly "(Kemp 1985, 67).
Darwin suggested that living things emerge by gradual evolution, and hoped that one day the fossil record would confirm its prediction, but that was not the case: Darwin was wrong. Day after day, the numerous fossils excavated all over the world have refuted the hypothesis of gradual change in supposedly chronological overlapping layers. Contrary to what Darwin expected, recent data reveal patterns of sudden onset (explosions) followed by long periods of little change Famous evolutionary paleontologist Niles Eldredge admitted in New Scientist magazine that:
"paleontologists since Darwin have been searching (largely in vain) for sequences of graduated fossil series that stand out as examples of the kind of global species transformation that Darwin imagined as the natural product of the evolutionary process. doubts - although it is an astonishing fact that... most species remain knowingly the same, virtually unchanged throughout their occurrence in geological strata of various ages "(Eldredge, 1986, p.55).
The absence of intermediate fossils is too obvious for evolutionists to conceal any longer. Evolutionary biologist Dr. David Woodruff of the University of California expressed in the journal Science the disappointment of evolutionists regarding the absence in the fossil record of transitional forms: "fossil species remain unchanged for most of their history and the record can not contain a single example of a meaningful transition. " (Woodruff, 1980, p.716).
So, in the face of so many admissions, we finally agree with evolutionary biologist Lynn Margulis when he says:
"There is no gradualism in the fossil record. [...] 'Punctuated equilibrium' was invented to describe the discontinuity in the emergence of new species. [...] Critics, [including critics SRABUCs], are right in the criticisms they make. [...] Evolutionary biologists believe that the evolutionary pattern is a tree. It's not! "(Teresi, 2011).
Examples of rapid speciation
There are several reports of the emergence of new "species" in periods ranging from tens to thousands of years. According to this information, speciation is a phenomenon that does not require millions of years to happen. US biologist Dr. James Gibson, Director of the Institute for Geoscience Research (GRI), an affiliate of Andrews University (USA), has described an example of real-time speciation: a new species of copepod formed in the Salton Sea in southern California in less than 30 years. "(Johnson, 1953, Gibson, 2002).
Several studies have reported that only between 10 and 36 years, different populations of lizards suffered morphological alterations that were significant enough to be considered new "species" (Morrell, 1997; Herrel et al., 2008). Other known examples of real-time speciation include bacteria (Shikano et al., 1990), flies (Huey et al., 2000), finches (Grant and Grant, 2006), frogs (Hoskin et al., 2005), beetles ( Halliburton and Gall, 1981) and plants (Groves and Groves, 1880; Foucaud, 1897; Marchant, 1963).
Dr. Gibson also provided examples of speciation in historical-archaeological time: "A population of green monkeys lived on the island of St Kitts in the Caribbean for less than 100 years, but developed morphological features equivalent to a new species." (Ashton et al. , 1979; Gibson, 2002). "Hawaii had no banana trees until about 1000 years ago, however there are native Hawaiian moths that only eat banana plants. These new species arose in less than 1000 years. "(Zimmerman, 1960, Gibson, 2002). Faced with these examples, Dr. Gibson concludes: "The capacity for rapid change is confirmed both by experimentation and by observation of nature" (Gibson, 2002).
Conclusion
Morphological patterns around the genus taxon (PMTG) identify with basic ancestral fossils buried in a recent catastrophism (SRABUC), since this model respects the fact of the fast speciation observed, which would require a very large proportional variability in the fossil record, if it represented the evolution in millions of years (PMS). In addition to this there is no such taxonomic variation, it is still confirmed by morphological stasis, repetition of the same species and 4229 genera of living fossils, a picture that reflects a sudden burial of living beings on the planet and not a supposed evolutionary history of Ma's life. Reports of speciation in history and archeology, capable of justifying all biodiversity in a short time, show us that we should have much more variability in the fossil record than there is, which, given the fact that evolution is we can make sure there was no time for her to act. The Cambrian explosion, the absence of fossil sample variability (fossil morphological stasis and taxonomic poverty) and lack of special radiation in the fossil record (diversification) up to the Pleistocene, tell us a story of a period of 1) rapid and ready appearance of forms 2) morphological permanence with a high number of species in a stable environment (fossil replication without evolutionary and adaptive environmental pressures, and without major catastrophes intercalating time), 3) living earthquake disaster evidenced by repetitions of the same fossil species (which (4) the presence of several different species united in the fossil record, fossils of immense continental vertebrates (which characterizes a large-scale disaster and high sedimentation rates), and 5) a drastic change in the environment generating the species' radiation (diversification) in recent strata in a sampled form (fossils and millions of species in today's biodiversity. "While 200 years ago, naturalists thought there were perhaps five or ten thousand species on Earth, current estimates ... put the value at ten or 20 million. It is often assumed that life has never been more diversified than it is today. " (Benton et al., 2007). These points raised the question of geochronology, and do not seek, as many scientific articles do, to justify ad hocs anachronistic anomalies than would be expected in paleontological facts, but highlight more independent interpretations of geochronology conventional, thus suggesting broad questioning of the time-scale, "absolute" time-dating, and especially the uniformitarian hypothesis that nothing accelerated radioactive decay by aging rocks during the supposed billions of years that have been modern scientific and dynamic thinking, even possessing perhaps hundreds of methods and refinements to accelerate radioactive decay and say, to age the rocks with voltages and environments which represent a paltry comparison to what occurred when immense cars collapsed on the earth where about 99, 8% were erased by d tectonic, maritime, erosive and sedimentary tectonics of the earth.
REFERÊNCIAS
1.
2.
3. Aaron M (2014). Baraminological Analysis of the Caseidae (Synapsida: Pelycosauria). Journal of Creation Theology and Science Series B: Life Sciences. 4:19-22.
4. Albrecht C, Wilke T (2008). Ancient Lake Ohrid: biodiversity and evolution. Hydrobiologia. 615(1):103-140.
5. Alisson E (2013). Arqueologia ajudará a desvendar origem da biodiversidade amazônica. Agência FAPESP. Disponível em: http://agencia.fapesp.br/arqueologia_ajudara_a_desvendar_origem_da_biodiversidade_amazonica/16937/
6. Ariza LM (2007). Evolution in a Petri Dish. Scientific American. 297(6):36. Disponível em: http://www.scientificamerican.com/article/evolution-in-a-petri-dish/
7. Ashton EH, Flinn RM, Griffiths RK, Moore WJ (1979). The results of geographic isolation on the teeth and skull of the Green monkey (Cercopithecus aethiopssabaeus) in St. Kitts – a multivariate retrospect. Journal of Zoology. 188(4):533-555.
8. Aw Kinderewitsch e Li Kitscha: Polewaja suschtschnost jadernoi fisiki - campo de Física Nuclear, Kiev: EKMO, 2003, páginas 263 -30
9. Baumgardner, Jonh (2005). «Radioisotopes and the Age of the Earth, Volume II». Institute for Creation Research. Consultado em 10 de setembro de 2017
10. Behe, M. June 1997). «Darwinism and design». Trends in Ecology & Evolution. 12 (6). 229 páginas. ISSN 0169-5347. PMID 2. (J1238050
11. Behe, Michael J. (February 2009). «Waiting longer for two mutations». Genetics. 181 (2): 819–820; author reply 821–822. ISSN 0016-6731. PMID 19189948. doi:10.1534/genetics.108.098905
12. Bell G (2013). Evolutionary rescue and the limits of adaptation. Philos Trans R Soc Lond B Biol Sci. 368(1610):20120080.
13. Benedict, John C.; Smith, Selena Y.; Specht, Chelsea D.; Collinson, Margaret E.; Leong-Škorničková, Jana; Parkinson, Dilworth Y.; Marone, Federica (2016). «Species diversity driven by morphological and ecological disparity: a case study of comparative seed morphology and anatomy across a large monocot order». AoB PLANTS. 8. ISSN 2041-2851. PMID 27594701
14. Benton, Michael J.; Emerson, Brent C. (1 de janeiro de 2007). «How Did Life Become so Diverse? the Dynamics of Diversification According to the Fossil Record and Molecular Phylogenetics». Palaeontology (em inglês). 50 (1): 23–40. ISSN 1475-4983. doi:10.1111/j.1475-4983.2006.00612.x
15. Berthault, G. : “Orogenesis: Cause of sedimentary formations” – Kazan Golovkinsy Stratigraphic Meeting, 2014, pp.19-20
16. Berthault, G. : “Orogenesis: cause of sedimentary formations” – “Open Journal of Geology“ ISSN 2161-7570.Vol 3, Number 28, April 2013.
17. Berthault G. : “Towards a Refoundation of Historical Geology” – “Georesources” 1(12) 2012, p.38, 39
18. Berthault, G., Lalomov, A. V. and Tugarova, M. A. : “Reconstruction of paleolithodynamic formation conditions of Cambrian-Ordovician sandstones in the Northwestern Russian platform” – “Lithology and Mineral Resources, 2011, Volume 46, Number 1, 60-70” (Springer Publishing site)
19. Berthault, G., Veksler A.B., Donenberg V.M. , Lalomov A. : “RESEARCH on EROSION OF CONSOLIDATED and semi-consolidated SOILS BY HIGH SPEED WATER FLOW” Izvestia.VNIIG., 2010, Vol. 257, pp.10-22. – (Russian original.)
20. Berthault, G : “Sedimentological Interpretation of the Tonto Group Stratigraphy (Grand Canyon Colorado River)” , Lithology and Mineral Resources 2004, Vol. 39, No 5. October 2004.
21. Berthault G., “Analysis of Main Principles of Stratigraphy on the Basis of Experimental Data”, Litol.Polezn.Iskop.2002, vol 37, no.5,pp 509-515 (Lithology and Mineral resources 2002 (fac-similé) (Engl.Transl.), vol.37, no.5, pp442-446), Journal of the Academy of Sciences of Russia.
22. Berthault, G., “Sedimentation of a Heterogranular Mixture. Experimental Lamination in Still and Running Water”, Compte rendu de l’Académie des Sciences 1988, vol. 306, Serie II, pp. 717–724.
23. Berthault, G., “Sedimentologie: Expériences sur la lamination des sédiments par granoclassement périodique postérieur au dépôt. Contribution a l’explication de la lamination dans nombre de sédiments et de roches sédimentaires”., Compte rendu de l’Académie des Sciences de Paris 1986 , vol. 303, Ser., 2, no. 17, pp. 1569-1574.
24. Bhullar BS (2017). Evolution: Catastrophe triggers diversification. Nature. 542 (7641):304-305.
25. Bosch, Fritz (1999). «Setting a Cosmic Clock with Highly Charged Ions». Physica Scripta (em inglês). T80 (A). ISSN 1402-4896. doi:10.1238/physica.topical.080a00028
26. Brand LR, Tang T (1991). Fossil vertebrate footprints in the Coconino Sandstone (Permian) of northern Arizona: Evidence for underwater origin. Geology. 19(12):1201-1204.
27. Brawn, Dan (2013). «The Origin of Earth's Radioactivity». Consultado em 24 de setembro de 2017
28. Bunch, Ted E.; Hermes, Robert E.; Moore, Andrew M. T.; Kennett, Douglas J.; Weaver, James C.; Wittke, James H.; DeCarli, Paul S.; Bischoff, James L.; Hillman, Gordon C. (10 de julho de 2012). «Very high-temperature impact melt products as evidence for cosmic airbursts and impacts 12,900 years ago». Proceedings of the National Academy of Sciences of the United States of America. 109 (28): E1903–1912. ISSN 1091-6490. PMID 22711809. doi:10.1073/pnas.1204453109
29. Carroll, R. L. (1992). «The Primary Radiation of Terrestrial Vertebrates». Annual Review of Earth and Planetary Sciences. 20 (1): 45–84. doi:10.1146/annurev.ea.20.050192.000401
30. Catalogue of Life (2016). Species 2000 & ITIS Catalogue of Life, 2016 Annual Checklist. Disponível em: http://www.catalogueoflife.org/col/info/ac
31. Cavanaugh DP, Sternberg RV (2004). Analysis of morphological groupings using ANOPA, a pattern recognition and multivariate statistical method: A case study involving centrarchid fishes. Journal of Biological Systems. 12(2):137–67.
32. Cavanaugh DP, Wood TC (2002). A Baraminological Analysis of the Tribe Heliantheaesensulato (Asteraceae) Using Analysis of Pattern (ANOPA). Occas. Papers of the BSG. 17(1):1-11.
33. Chadwick A, Spencer L (2006). Turner Preliminary depositional model for an Upper Cretaceous Edmontosaurusbonebed. Journal of Vertebrate paleontology. 26:49A.
34. Crabtree GR (2013a). Our fragile intellect. Part I. Trends in Genetics. 29(1):1-3.
35. Crabtree GR (2013b). Our fragile intellect. Part II. Trends in Genetics. 29(1):3-5.
36. Crow JF (1997). The high spontaneous mutation rate: is it a health risk? Proc Natl AcadSci U S A. 94(16):8380-6.
37. Darwin C (1861). On the Origin of Species. 3. Ed. London: Murray. capítulo 14.
38. de Paula MO (2009). Podem os criacionistas aceitar a origem de novas espécies? Earth History Research Center. Disponível em: http://origins.swau.edu/papers/evol/marcia1/defaultp.html
39. de Queiroz K (2005). Ernst Mayr and the modern concept of species. Proc Natl AcadSci U S A. 102 Suppl 1:6600-7.
40. Dilly, R., Berthault, G.: “Orogenesis: Cause of sedimentary formations” – The Russian Academy of Sciences scientific Council on Lithology and Minerals in Sedimentary Formations – VIII All-Russian Lithological Meeting (Moscow, 27-30 October 2015), Tome II, pp. 162-164
41. Dolgin E (2009). Human mutation rate revealed. Nature News (27 Ago. 2009). Disponível em:http://www.nature.com/news/2009/090827/full/news.2009.864.html
42. Donnelley, Strachan (July 2002). «Natural responsibilities. Philosophy, biology, and ethics in Ernst Mayr and Hans Jonas». The Hastings Center Report. 32(4): 36–43. ISSN 0093-0334. PMID 12362522
43. Eakin CM (2014). Lamarck was partially right—and that is good for corals. Science. 344(6186):798-9.
44. Eldredge N, Stanley SM (1984). Living Fossils: Introduction to the Casebook. New York/Berlin: Springer-Verlag pp. 266-270.
45. Eldredge N (1986). Progress in Evolution? New Scientist. 110:54-57.
46. Eyre-Walker A, Keightley PD (1999). High genomic deleterious mutation rates in hominids. Nature. 397(6717):344-7.
47. Ezard, Thomas H. G.; Quental, Tiago B.; Benton, Michael J. (5 de abril de 2016). «The challenges to inferring the regulators of biodiversity in deep time». Phil. Trans. R. Soc. B(em inglês). 371 (1691). 20150216 páginas. ISSN 0962-8436. PMID 26977058. doi:10.1098/rstb.2015.0216
48. Grant, B. Rosemary; Grant, Peter R. (3 de março de 2017). «Watching speciation in action». Science (New York, N.Y.). 355 (6328): 910–911. ISSN 1095-9203. PMID 28254901. doi:10.1126/science.aam6411.
49. Folha de S. Paulo (2006). Pássaros exibem seleção natural em tempo real. Folha de São Paulo (14/07/2006). Disponível em: http://www1.folha.uol.com.br/folha/ciencia/ult306u14848.shtml
50. Fondon JW, Garner HR (2004). Molecular origins of rapid and continuous morphological evolution. Proc Natl AcadSci U S A. 101(52):18058-63.
51. Foote, Mike (1993/04). «Discordance and concordance between morphological and taxonomic diversity». Paleobiology. 19 (2): 185–204. ISSN 0094-8373. doi:10.1017/S0094837300015864
52. Foucaud (1897). Un Spartinainédit. Ann SocSci Nat Char Inf. 32:220–222.
53. Frair W (2000). Baraminology: Classification of Created Organisms. CRSQ Quarterly. 37(2):82-91.
54. Freeman S, Herron JC (2009). Análise evolutiva. 4. ed. orto Alegre: Artmed. 848p.
55. Fu W, et al (2013). Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature. 493(7431):216-20.
56. Futuyma, Douglas J. (July 2010). «Evolutionary constraint and ecological consequences». Evolution; International Journal of Organic Evolution. 64(7): 1865–1884. ISSN 1558-5646. PMID 20659157. doi:10.1111/j.1558-5646.2010.00960.x
57. Furness, Andrew I.; Lee, Kevin; Reznick, David N. (June 2015). «Adaptation in a variable environment: Phenotypic plasticity and bet-hedging during egg diapause and hatching in an annual killifish». Evolution; International Journal of Organic Evolution. 69 (6): 1461–1475. ISSN 1558-5646. PMID 25908306. doi:10.1111/evo.12669
58. Gentry, R.V. 1970. "Giant Radioactive Halos: Indicators of Unknown Alpha-Radioactivity?" Science 169, 670.
59. Gentry, R.V. 1971. "Radiohalos: Some Unique Pb Isotope Ratios and Unknown Alpha Radioactivity." Science 173, 727.
60. Gentry, R.V. 1973. "Radioactive Halos." Annual Review of Nuclear Science 23, 347. Acessado em 24/09/2017 em http://www.robertvgentry.com/
61. Gentry, R.V. 1974. "Radiohalos in Radiochronological and Cosmological Perspective." Science 184, 62. Acessado em 24/09/2017 em http://www.robertvgentry.com/
62. Gentry, R.V. 1977. "Mystery of the Radiohalos." Research Communications NETWORK, Breakthrough Report, February 10, 1977. Acessado em 24/09/2017 em http://www.robertvgentry.com/
63. Gentry, R.V. 1978a. "Are Any Unusual Radiohalos Evidence for SHE?" International Symposium on Superheavy Elements, Lubbock, Texas. New York: Pergamon Press.
64. Gentry, R.V. 1978b. "Implications on Unknown Radioactivity of Giant and Dwarf Haloes in Scandinavian Rocks." Nature 274, 457.
65. Gentry, R.V. 1978c. "Reinvestigation of the α Activity of Conway Granite." Nature 273, 217. PDF
66. Gentry, R.V. 1979. "Time: Measured Responses." EOS Transactions of the American Geophysical Union 60, 474. Acessado em 24/09/2017 em http://www.robertvgentry.com/
67. Gentry, R.V. 1980. "Polonium Halos." EOS Transactions of the American Geophysical Union 61, 514.
68. Gentry, R.V. et al. 1973. "Ion Microprobe Confirmation of Pb Isotope Ratios and Search for Isomer Precursors in Polonium Radiohalos." Nature 244, 282.
69. Gentry, R.V. et al. 1974. "'Spectacle' Array of Po-210 Halo Radiocentres in Biotite: A Nuclear Geophysical Enigma." Acessado em 24/09/2017 em http://www.robertvgentry.com/
70. Gentry, R.V. et al. 1976a. "Radiohalos and Coalified Wood: New Evidence Relating to the Time of Uranium Introduction and Coalification." Science 194, 315 . Acessado em 24/09/2017 em http://www.robertvgentry.com/
71. Ghalambor, Cameron K.; Hoke, Kim L.; Ruell, Emily W.; Fischer, Eva K.; Reznick, David N.; Hughes, Kimberly A. (17 de setembro de 2015). «Non-adaptive plasticity potentiates rapid adaptive evolution of gene expression in nature». Nature (em inglês). 525 (7569): 372–375. doi:10.1038/nature15256
72. Gibson LJ (2002). A similaridade biológica e a teoria da evolução. In: 4º Encontro Nacional de Criacionistas, 24 a 28 de janeiro de 2002, São Paulo. Anais... São Paulo: Centro Universitário Adventista, campus São Paulo.
73. Gibson, L. J. --- Fossil Patterns: A Classification and Evaluation». www.grisda.org. Consultado em 10 de setembro de 2017
74. Gorodezki V, 2005. Deactivation unit for radioactive materials, comprises capacitor with curved mantle surfaces, connected to controllable generator DE 10354251 A1.Acessado em 24/09/2017 em http://www.google.com.na/patents/DE10354251A1?cl=en&hl=pt-BR
75. Gould SJ (1981). Evolution as Fact and Theory, Discover magazine 1981; 2(5):34-37; Reimpresso com permissãoem: Gould SJ. Hen's Teeth and Horse's Toes. New York: W. W. Norton & Company, 1994, pp. 253-262.
76. Grant PR, Grant BR (2006). Evolution of Character Displacement in Darwin's Finches. Science. 313(5784):224-6.
77. Groves H, Groves J (1880). Spartinatownsendiinobis. Rep Bot SocExch Club Br Id. 1:37.
78. Donald R. Lowe, Gary R. Byerly, Frank T. Kyte, Alexander Shukolyukov, Frank Asaro e Alexandra Krull. Astrobiologia. Julho de 2004, 3 (1): 7-48. https://doi.org/10.1089/153110703321632408
79. Halliburton R, Gall GAE (1981). Disruptive selection and assortative mating in Triboliumcastaneum. Evolution. 35:829-843.
80. Herrel A, et al (2008). Rapid large-scale evolutionary divergence in morphology and performance associated with exploitation of a different dietary resource. Proc Natl AcadSci U S A. 105(12):4792-5.
81. Hesslow, L.; Embréus, O.; Stahl, A.; DuBois, T. C.; Papp, G.; Newton, S. L.; Fülöp, T. (23 de junho de 2017). «Effect of Partially Screened Nuclei on Fast-Electron Dynamics». Physical Review Letters. 118 (25). 255001 páginas. ISSN 1079-7114. PMID 28696736. doi:10.1103/PhysRevLett.118.255001
82. Horner JR, Goodwin MB (2009). Extreme Cranial Ontogeny in the Upper Cretaceous Dinosaur Pachycephalosaurus. PLoS One. 4(10):e7626.
83. Hoskin CJ1, Higgie M, McDonald KR, Moritz C (2005). Reinforcement drives rapid allopatric speciation. Nature. 437(7063):1353-6.
84. Huey RB, et al (2000). Rapid Evolution of a Geographic Cline in Size in an Introduced Fly. Science. 287(5451):308-309.
85. I Have A List"». Eye on the ICR (em inglês). 31 de julho de 2011
86. Izumi, Y.; Tomita, H.; Nakayama, Y.; Hayashi, S.; Morishima, K.; Isobe, M.; Cheon, M. S.; Ogawa, K.; Nishitani, T. (November 2016). «Development of fast neutron pinhole camera using nuclear emulsion for neutron emission profile measurement in KSTAR». The Review of Scientific Instruments. 87 (11): 11D840. ISSN 1089-7623. PMID 27910454. doi:10.1063/1.4963866
87. Jerlström P (2000). Is the evolutionary tree turning into a creationist orchard? Journal of Creation. 14(2):11–13.
88. Johnson MW (1953). The copepod Cyclops dimorphus Kiefer from the Salton Sea. American Midland Naturalist. 49:188-192.
89. Julien, P.Y., Lan, Y., and Berthault, G., “Experiments on Stratification of Heterogeneous Sand Mixtures”, Bulletin Société Géologique de France, 1993, vol. 164, no. 5, pp. 649–660.
90. Junker R, Scherer S (1996). Evollução: um livro texto crítico. 4. Ed. Brasília, DF: Sociedade Criacionista Brasileira.
91. Kahle H (1999). Evolution: IrrwegmodernerNaturwissenschaft? 4. Ed. Bielefeld: Mindt, Edeltraud. 200p.
92. Keightley PD (2012). Rates and Fitness Consequences of New Mutations in Humans. Genetics. 190(2):295-304.
93. Kemp TS (1985). A Fresh Look at the Fossil Record. New Scientist. 108(1485):66-67.
94. Kennett, James P.; Kennett, Douglas J.; Culleton, Brendan J.; Aura Tortosa, J. Emili; Bischoff, James L.; Bunch, Ted E.; Daniel, I. Randolph; Erlandson, Jon M.; Ferraro, David (11 de agosto de 2015). «Bayesian chronological analyses consistent with synchronous age of 12,835-12,735 Cal B.P. for Younger Dryas boundary on four continents». Proceedings of the National Academy of Sciences of the United States of America. 112 (32): E4344–4353. ISSN 1091-6490. PMID 26216981. doi:10.1073/pnas.1507146112
95. Kennett, James P.; Kennett, Douglas J.; Culleton, Brendan J.; Aura Tortosa, J. Emili; Bischoff, James L.; Bunch, Ted E.; Daniel, I. Randolph; Erlandson, Jon M.; Ferraro, David (11 de agosto de 2015). «Bayesian chronological analyses consistent with synchronous age of 12,835-12,735 Cal B.P. for Younger Dryas boundary on four continents». Proceedings of the National Academy of Sciences of the United States of America. 112 (32): E4344–4353. ISSN 1091-6490. PMID 26216981. doi:10.1073/pnas.1507146112
96. Kuhn, Joseph A. (2012-1). «Dissecting Darwinism». Proceedings (Baylor University. Medical Center). 25 (1): 41–47. ISSN 0899-8280. PMID 22275784
97. Lacy, Robert C. (1 de agosto de 1987). «Loss of Genetic Diversity from Managed Populations: Interacting Effects of Drift, Mutation, Immigration, Selection, and Population Subdivision». Conservation Biology (em inglês). 1 (2): 143–158. ISSN 1523-1739. doi:10.1111/j.1523-1739.1987.tb00023.x
98. Lalomov, A., Berthault G., Tugarova, M., Isotov V., Sitdikova L.: “Reconstruction of sedimentary conditions of Middle Permian Kama-Ural basin studied by N.A.Golovkinsky” – Kazan Golovkinsy Stratigraphic Meeting, 2014, pp.53-54
99. Lalomov, A. : “Reconstruction of Paleohydrodynamic Conditions during the Formation of Upper Jurassic Conglomerates of the Crimean Peninsula”, Lithology and Mineral Resources, 2007, Vol. 42, No. 3, pp. 268–280
100. Lavoué, Sébastien; Miya, Masaki; Arnegard, Matthew E.; McIntyre, Peter B.; Mamonekene, Victor; Nishida, Mutsumi (7 de abril de 2011). «Remarkable morphological stasis in an extant vertebrate despite tens of millions of years of divergence». Proceedings of the Royal Society B: Biological Sciences. 278 (1708): 1003–1008. ISSN 0962-8452. PMID 20880884.
101. Lee, Kanani K. M.; Steinle-Neumann, Gerd (30 de março de 2008). «Ab-initio study of the effects of pressure and chemistry on the electron-capture radioactive decay constants of 7Be, 22Na and 40K». Earth and Planetary Science Letters. 267 (3): 628–636. doi:10.1016/j.epsl.2007.12.014
102. Levinton JS, Chris MS (1980). A Critique of the Punctuated Equilibria Model and Implications for the Detection of Speciation in the Fossil Record. Syst Zool. 29(2):130–142.
103. Lightner JK (2016). Variation after the Flood. Creation Matters. 21(2):6-7.
104. Locey KJ, Lennon JT (2016). Scaling laws predict global microbial diversity. Proc Natl AcadSci U S A. 113(21):5970-5.
105. Lönnig, Wolf-Ekkehard (2004). «Dynamic genomes, morphological stasis, and the origin of irreducible complexity». Max-Planck-Institut for Plant Breeding Research. Consultado em 9 de setembro de 2017
106. Lopes RJ (2015). Afobação faz cientistas classificarem fósseis de símios como hominídeos. Folha de S. Paulo. Disponível em: http://m.folha.uol.com.br/ciencia/2015/09/1676679-no-afa-cientistas-tem-classificado-fosseis-de-simios-como-hominideos.shtml?mobile
107. Lynch M (2010). Rate, molecular spectrum, and consequences of human mutation.Proc Natl AcadSci U S A. 107(3):961-8.
108. Marchant CJ (1963). Corrected chromosome numbers for Spartina x townsendii and its parent species. Nature. 199:929.
109. Marsh FL (1941). Fundamental Biology. Lincoln, NE: Published by the Author.
110. Marsh FL (1978). Variation And Fixity Among Living Things. A New Biological Principle.CRS Quarterly. 15(2):115-118.
111. Martens K (1997). Speciation in ancient lakes. Trends EcolEvol. 12(5):177-82.
112. Mendonça de Souza, S. M. F., Rodrigues-Carvalho, C., Silva, H. P., & Locks, M. (2006). Revisitando a discussão sobre o Quaternário de Lagoa Santa e o povoamento das Américas: 160 anos de debates científicos. In H. P. Silva & C. Rodrigues-Carvalho (Eds.), Nossa origem. O povoamento das Américas – visões multidisciplinares (pp. 19–43). Rio de Janeiro: Viera & Lentz.
113. Minoletti, Fabrice; Hermoso, Michaël; Gressier, Vincent (2009). «Separation of sedimentary micron-sized particles for palaeoceanography and calcareous nannoplankton biogeochemistry». Nature Protocols. 4 (1): 14–24. ISSN 1750-2799. PMID 19131952. doi:10.1038/nprot.2008.200
114. Mora C, Tittensor DP, Adl S, Simpson AG, Worm B (2011). How many species are there on Earth and in the ocean? PLoS Biol. 9(8):e1001127.
115. Morrell V (1997). Catching Lizards in the Act of Adapting. Science. 276(5313):682-683.
116. Nachman MW, Crowell SL (2000). Estimate of the Mutation Rate per Nucleotide in Humans. Genetics. 156(1):297-304.
117. Nagalingum NS, et al (2011). Recent Synchronous Radiation of a Living Fossil. Science. 334 (6057): 796-799.
118. NASA Astrobiology Institute». nai.nasa.gov. Consultado em 24 de setembro de 201
119. Prüfer K, et al (2013). The complete genome sequence of a Neanderthal from the Altai Mountains. Nature. 505:43-49.
120. ReMine WJ (1993). The Biotic Message. St Paul, MN: St Paul Science.
121. Reinhard Junker, Siegfried Scherer: Evolution – Ein kritisches Lehrbuch. Gießen, ISBN 3-921046-10-6
122. Ridley M (2006). Evolução. 3. ed. Porto Alegre: Artmed. 752p.
123. Robinson DA, Cavanaugh DP (1998). A quantitative approach to baraminology with examples from the primates. CRSQ Quarterly. 34:196–208.
124. Robinson DA (1997). A mitochondrial DNA analysis of the testudineapobaramin. Creation Research Society Quarterly. 33:262–72.
125. Romer AS (1966). Vertebrate Paleontology. 3rd Ed. Chicago: University of Chicago Press, pp. 347–396. In: Denton M. Evolution: A Theory in Crisis. Bethesda: Adler & Adler, 1986, p. 202.
126. Sadava D, Heller HC, Orians GH, Purves WK, Hillis DM (2009). Vida: A Ciência da Biologia. 8. Ed., v. 2. Porto Alegre: EditoraArtmed.
127. Sadler, Peter M. (1 de setembro de 1981). «Sediment Accumulation Rates and the Completeness of Stratigraphic Sections». The Journal of Geology. 89 (5): 569–584. ISSN 0022-1376. doi:10.1086/628623
128. Sanford, John & Baumgardner, John & Brewer, Wesley & Gibson, Paul & Remine, Walter. (2008). Using Numerical Simulation to Test the Validity of Neo-Darwinian Theory.
129. Sanford John C. (2014). Genetic Entropy. 4. ed. New York: FMS Publications.
130. Scherer S (1993). Typen des Lebens. Berlin: Pascal.
131. Schwartz JH, Tattersall I (2015). Defining the genus Homo. Science. 349(6251):931-2.
132. Senter P (2010). Using creation science to demonstrate evolution: application of a creationist method for visualizing gaps in the fossil record to a phylogenetic study of coelurosaurian dinosaurs. Journal of Evolutionary Biology. 23:1732–1743.
133. Sepkoski, J. J. (1993). «Ten years in the library: new data confirm paleontological patterns». Paleobiology. 19 (1): 43–51. ISSN 0094-8373. PMID 11538041
134. Sepkoski, David (2013). «Towards "a natural history of data": evolving practices and epistemologies of data in paleontology, 1800-2000». Journal of the History of Biology. 46 (3): 401–444. ISSN 0022-5010. PMID 22956055. doi:10.1007/s10739-012-9336-6
135. Shabalina SA, Ogurtsov AY, Kondrashov VA, Kondrashov AS (2001). Selective constraint in intergenic regions of human and mouse genomes. Trends Genet. 17(7):373-6.
136. Schopf JW. 1992. Patterns of Proterozoic microfossil diversity: an initial, tentative, analysis. In: Schopf and Klein, p 529-552; (b) Walter MR, Grotzinger JP, Schopf JW. 1992. Proterozoic stromatolites. In: Schopf and Klein, p 253-260
137. Shikano S, Luckinbill LS, Kurihara Y (1990). Changes of traits in a bacterial population associated with protozoal predation. Microbial Ecology. 20:75-84.
138. Snelling A (1997). Sedimentation experiments: Nature finally catches up! Journal of Creation. 11(2):125-126.
139. Souza Jr NN (2008). Uma breve história da terra. Brasília, DF: Sociedade Criacionista Brasileira.
140. Spray, John G.; Kelley, Simon P.; Rowley, David B. (12 de março de 1998). «Evidence for a late Triassic multiple impact event on Earth». Nature (em inglês). 392 (6672): 171–173. ISSN 0028-0836. doi:10.1038/32397
141. Teresi D (2011). Discover Interview: Lynn Margulis Says She's Not Controversial, She's Right. Discover Magazine, p. 68.
142. Trivedi BP (2000). Island mice may evolve faster: From one species to six. Genome News Network. Disponível em: http://www.genomenewsnetwork.org/articles/04_00/island_mice.shtml
143. Van Bocxlaer B, Hunt G (2013). Morphological stasis in an ongoing gastropod radiation from Lake Malawi. PNAS. 110(34):13892-13897.
144. Voje KL (2016). Tempo does not correlate with mode in the fossil record. Evolution. 70(12):2678-2689.
145. Xie, Xufei; Chen, Zhongjing; Peng, Xingyu; Yuan, Xi; Zhang, Xing; Gorini, Giuseppe; Cui, Zhiqiang; Du, Tengfei; Hu, Zhimeng (October 2014). «Neutron emission measurement at the HL-2A tokamak device with a liquid scintillation detector». The Review of Scientific Instruments. 85 (10). 103506 páginas. ISSN 1089-7623. PMID 25362392. doi:10.1063/1.4896120
146. Weyrich A, et al (2016). Paternal intergenerational epigenetic response to heat exposure in male Wild guinea pigs. Mol Ecol. 25:1729-1740.
147. Whitmore J (2013a). Temporal Patterns in 'Living Fossils'. In: 4th Annual Research and Scholarship Symposium. Cedarville, OH. Anais... Cedarville, OH: Cedarville University. Disponível em: http://digitalcommons.cedarville.edu/research_scholarship_symposium/2013/poster_presentations/22/
148. Whitmore J (2013b). What about living fossils? Capítulo 12, pp.143-150. In: Ham K (Ed.). The New Answers Book, v. 4. Green Forest: Master Books.
149. Wills, Matthew A.; Briggs, Derek E. G.; Fortey, Richard A. (1994/04). «Disparity as an evolutionary index: a comparison of Cambrian and Recent arthropods». Paleobiology. 20 (2): 93–130. ISSN 0094-8373. doi:10.1017/S009483730001263X
150. Williamson PG (1981). Morphological Stasis and Developmental Constraint: Real Problems for Neo-Darwinism. Nature. 294:214-2015.
151. Wilmer JW, et al (2011). Catastrophic Floods May Pave the Way for Increased Genetic Diversity in Endemic Artesian Spring Snail Populations. PLoS One. 6(12):e28645.
152. Wilson G (2010). Classic Multidimensional Scaling isn’t the Sine Qua Non of Baraminology. Answers in Genesis. Disponível em: https://answersingenesis.org/creation-science/baraminology/classic-multidimensional-scaling-and-baraminology/
153. Wise KP (1989) The Estimation of True Taxonomic Durations from Fossil Occurrence Data; University Microfilms, 1989.
154. Wise KP (1990). Baraminology: a young-earth creation biosystematic method. In: Walsh RE (Ed.). Proceedings of the Second International Conference on Creationism. Volume II, Technical Symposium, pp. 345–360. Creation Science Fellowship.
155. Wise KP (1992). Practical baraminology. Creation ex Nihilo Technical Journal. 6(2): 122–37.
156. Wise KP (2010) Relatório Gmail [8.7 milion x 300.000]. Mensagem recebida por <sodre.siman@gmail.com> em 13 out. 2015
157. Wittke, James H.; Weaver, James C.; Bunch, Ted E.; Kennett, James P.; Kennett, Douglas J.; Moore, Andrew M. T.; Hillman, Gordon C.; Tankersley, Kenneth B.; Goodyear, Albert C. (4 de junho de 2013). «Evidence for deposition of 10 million tonnes of impact spherules across four continents 12,800 y ago». Proceedings of the National Academy of Sciences of the United States of America. 110 (23): E2088–2097. ISSN 1091-6490. PMID 23690611. doi:10.1073/pnas.1301760110
158. Wood TC, Cavanaugh DP (2001). A baraminological analysis of subtribe Flaveriinae (Asteraceae: Helenieae) and the origin of biological complexity. Origins. 52:7-27.
159. Wood TC, Wise KP, Sanders R, Doran N (2003). A Refined Baramin Concept. Occas. Papers of the BSG. 3:1-14.
160. Wood TC (2001). BDIST software, v. 1.0. Center for Origins Research and Education, Bryan College. Distributed by the author.
161. Wood TC (2002). A baraminology tutorial with examples from the grasses (Poaceae). Journal of Creation. 16(1):15–25.
162. Wood TC (2005). Visualizing baraminic distances using classical multidimensional scaling. Origins. 57:9-29.
163. Wood TC (2010). Baraminological Analysis Places Homo habilis, Homo rudolfensis, and Australopithecus sediba in the Human Holobaramin. Answers Research Journal 3:71–90.
164. Wood TC (2011). Using creation science to demonstrate evolution? Senter’s strategy revisited. Journal of Evolutionary Biology. 24(4):914–918.
165. Woodmorappe J (2000). The Karoo vertebrate nonproblem: 800 billion fossils or not. CEN Technical Journal. 14(2):47-49.
166. Woodruff DS (1980). Evolution: The Paleobiological View. Science. 208(4445):716-717.
167. Zhang, Y. P.; Yang, J. W.; Liu, Yi; Fan, T. S.; Luo, X. B.; Yuan, G. L.; Zhang, P. F.; Xie, X. F.; Song, X. Y. (June 2016). «Development of the radial neutron camera system for the HL-2A tokamak». The Review of Scientific Instruments. 87 (6). 063503 páginas. ISSN 1089-7623. PMID 27370450. doi:10.1063/1.4953109
168. Zellner, Nicolle E. B. (1 de setembro de 2017). «Cataclysm No More: New Views on the Timing and Delivery of Lunar Impactors». Origins of Life and Evolution of Biospheres (em inglês). 47 (3): 261–280. ISSN 0169-6149. doi:10.1007/s11084-017-9536-3
169. Zimmerman EC (1960). Possible evidence of rapid evolution in Hawaiian moths. Evolution. 14:137-138.

